Boehringer Ingelheim:
Site-To-Site Transfer of Investigational Medicinal Product
The Challenge:
Operating in a regulated industry, Boehringer Ingelheim had to follow strict, FDA-monitored procedures in many areas. Compliance training, such as this course covering site to site transfer of investigational medicinal products, must be completed successfully by company employees with associated job responsibilities.
The Solution:
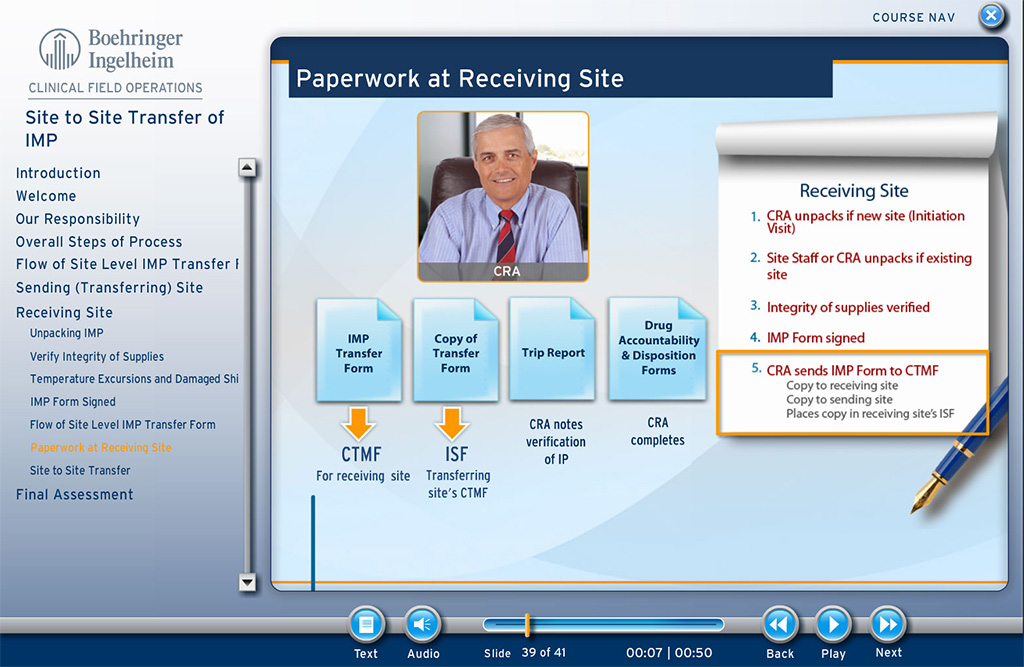
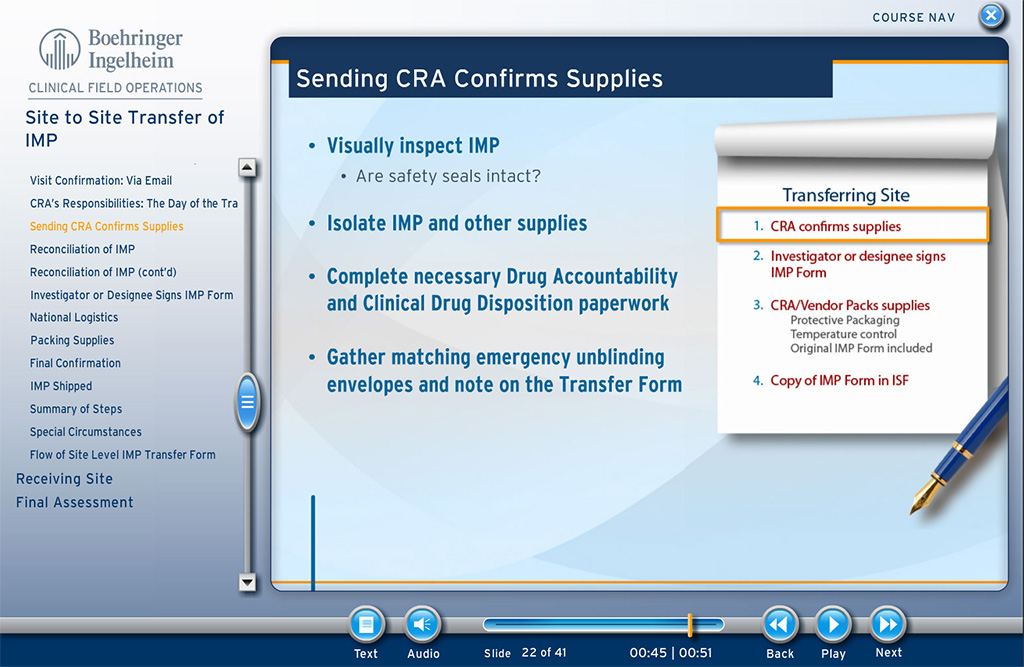
The course teaches the involved individuals about the required processes, involved individuals, typical timelines, and other aspects for transferring investigational medicinal product from one site to another, in those instances where such transfer is necessary. The course covers the procedures and also the specific forms needed for FDA compliance.
What our clients say about us
“They are quick to understand and adapt as the specs have evolved over time. Being flexible and responsive is a huge benefit. Because a lot of clients don’t know what they need at the outset, the Illumina team is good at co-creating that solution and making informative recommendations.” Rebecca Jackson Stoeckle Deputy Center Director, Center for Research on High Risk Behavior Health and Human Development Division, Education Development Center